Quantitative phase imaging (QPI) has emerged as an important tool in biomedicine that yields unprecedented insight into internal cellular structures, and which allows researchers to study cell nanoarchitecture, mass transport and cell membrane fluctuations for various biomedical applications. However, QPI has been limited to thin samples, typically the thickness of a single cell. To overcome this significant barrier, we developed quantitative oblique back-illumination microscopy (qOBM) [1,2], which provides the same rich level of quantitative insight provided by QPI but tomographically in thick scattering samples, including human tissue, which has significant implications for many biomedical and clinical applications.
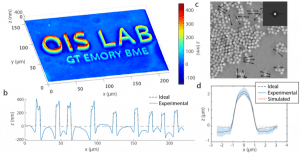
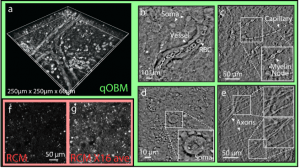
qOBM uses a convectional bright field microscope geometry with low-cost LED light sources in epi-mode, and leverages multiple-scattering within a thick sample to generate a virtual light source from within. By modeling the scattering process, robust estimates of the transfer function of the system can be obtained which ultimately leads to 3D quantitative phase imaging in real-time using a simple, low-cost optical microscope. Figure 1 shows the quantitative capabilities of qOBM, and Fig. 2 shows examples images of freshly excised mouse brain. A comparison to image acquired with reflectance confocal microcopy (RCM) of the same sample with equal acquisition times or X16 more averaging is also shown in Fig. 2. Indeed, the level of cellular and subcellular detail provided by this novel technology is unprecedented for label-free, tomographic tissue imaging (without resorting to expensive and complex nonlinear methods). Plus, qOBM has access to the same rich biophysical information as QPI, but in arbitrarily thick samples which is very significant.
qOMB is now being explored as a tool to monitor cell manufacturing processes [3,4], image 3D organoids [1], guide neurosurgery using a handheld qOBM probe, and much more [5,6].
Reference:
- P. Ledwig and F. E. Robles, “Epi-mode tomographic quantitative phase imaging in thick scattering samples,” Biomed Opt Express 10(7), 3605 (2019).
- P. Ledwig and F. E. Robles, “Quantitative 3D refractive index tomography of opaque samples in epi-mode,” Optica 8(1), 6 (2020).
- P. Ledwig, M. Sghayyer, J. Kurtzberg, and F. E. Robles, “Dual-wavelength oblique back-illumination microscopy for the non-invasive imaging and quantification of blood in collection and storage bags,” Biomed Opt Express 9(6), 2743 (2018).
- P. Casteleiro Costa, P. Ledwig, A. Bergquist, J. Kurtzberg, and F. E. Robles, “Noninvasive white blood cell quantification in umbilical cord blood collection bags with quantitative oblique back‐illumination microscopy,” Transfusion (2020).
- P. Casteleiro Costa, Z. Guang, P. Ledwig, Z. Zhang, S. Neill, J. J. Olson, and F. E. Robles, “Towards in-vivo label-free detection of brain tumor margins with epi-illumination tomographic quantitative phase imaging,” Biomed Opt Express 12(3), 1621 (2021).
- Z. Guang, P. Ledwig, P. C. Costa, C. Filan, and F. E. Robles, “Optimization of a flexible fiber-optic probe for epi-mode quantitative phase imaging,” Opt Express 30(11), 17713 (2022).