In general, nonlinear phenomena offer a wider source of physical mechanisms that can be used to probe biophysical and biochemical properties of endogenous biological species, compared to linear phenomena. The most widely used nonlinear technologies utilize interactions that generate new optical frequencies from the excitation light. Multiphoton fluorescence (MPF) microscopy, for instance, uses pulsed laser light in the near IR region of the spectrum (e.g., 800 nm) to excite fluorophores via a two- or more photon process, and detects emitted fluorescent light at lower wavelengths (higher energy) that is produced only at the focal region (see figure below). The unique properties of MPF enable deeper penetration depths and reduced sample damage compared to its linear counter part [1].
.
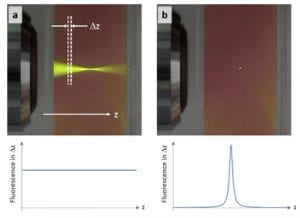
Unfortunately, there are only a few endogenous fluorophores, limiting the capabilities of MPF for label-free biomedical imaging. To gain access to the many other types on nonlinear interactions, pump-probe or transient spectroscopy–a method routinely used in physical chemistry–was applied to microscopy. Pump-probe microscopy gives access to many other types light-matter interactions (e.g., ground-state bleaching, stimulated emission, cross phase modulation), ultimately providing a large parameter space that yields highly specific molecular information. The figure below summarized the nonlinear interactions available for image contrast [2].
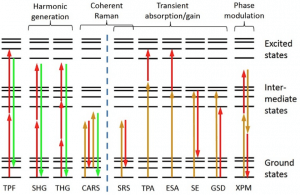
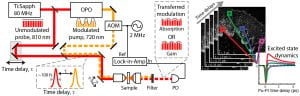
Pump-probe microscopy relies on detecting small amplitude changes in a probe beam when the pump is present compared to when it is absent. These changes are typically very small (~1E-3 to 1E-6 relative intensity change), and can be buried below the noise level of the pulsed laser light source. To overcome this limitation transfer modulation schemes are used, in which the pump laser is modulated at high rates (>1 MHz where laser noise is significantly reduced), and the modulation imparted on the probe beam after a nonlinear interaction is detected with a lock in amplifier. Because the pulses are typically ~100 femtoseconds in duration, this method is also able to map out the ultrafast electronic life-times of the excited states by varying the time-delay between the pump and probe [3-4]. This can provide highly specific molecular information.
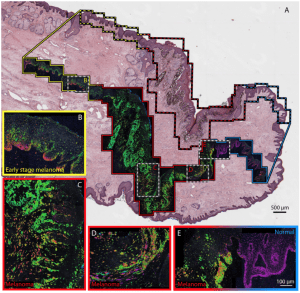
Pump-probe microscopy has been used to identify different types of melanins based on the molecules’ excited state photodynamics, with subcellular spatial resolution [3-4]. The detected nonlinear dynamics provide information regarding the pigments’ ion content, aggregate size, oxidative stress, and type (eu-/pheo- melanin) [5]. This method has found use in analyzing pigmented lesions for melanoma detection [4] and staging in excised human biopsy specimens [6] and animal models in vivo [7]. The figure below shows an example image of a thin tissue biopsy, where the ultrafast, nonlinear molecular signatures of the melanin can be used to identify cancerous (melanoma) regions.
References:
[1] Zipfel, W. R., Williams, R. M., & Webb, W. W. (2003). Nonlinear magic: multiphoton microscopy in the biosciences. Nature Biotechnology, 21(11), 1369–1377. http://doi.org/10.1038/nbt899
[2] Fischer, M. C., Wilson, J. W., Robles, F. E., & Warren, W. S. (2016). Invited Review Article: Pump-probe microscopy. The Review of Scientific Instruments, 87(3), 031101. http://doi.org/10.1063/1.4943211
[3] Fu, D., Ye, T., Matthews, T. E., Yurtsever, G., & Warren, W. S. (2007). Two-color, two-photon, and excited-state absorption microscopy. Journal of Biomedical Optics, 12(5), 054004. http://doi.org/10.1117/1.2780173
[4] Matthews, T. E., Piletic, I. R., Selim, M. A., Simpson, M. J., & Warren, W. S. (2011). Pump-probe imaging differentiates melanoma from melanocytic nevi. Science Translational Medicine. http://doi.org/10.1126/scitranslmed.3001604
[5] Simpson, M. J., Wilson, J. W., Robles, F. E., Dall, C. P., Glass, K., Simon, J. D., & Warren, W. S. (2014). Near-Infrared Excited State Dynamics of Melanins: The Effects of Iron Content, Photo-Damage, Chemical Oxidation, and Aggregate Size. The Journal of Physical Chemistry A, 118(6), 993–1003. http://doi.org/10.1021/jp4107475
[6] Robles, F. E., Deb, S., Wilson, J. W., Gainey, C. S., Selim, M. A., Mosca, P. J., et al. (2015). Pump-probe imaging of pigmented cutaneous melanoma primary lesions gives insight into metastatic potential. Biomedical Optics Express, 6(9), 3631–3645. http://doi.org/10.1364/BOE.6.003631
[7] Wilson, J. W., Degan, S., Gainey, C. S., Mitropoulos, T., Simpson, M. J., Zhang, J. Y., & Warren, W. S. (2015). Comparing in vivo pump–probe and multiphoton fluorescence microscopy of melanoma and pigmented lesions. Journal of Biomedical Optics, 20(5), 051012. http://doi.org/10.1117/1.JBO.20.5.051012