The wavelength-dependent behavior of light scattered by living cells reveals structural and functional information that is not readily available with traditional microscopy methods. For example, periodic fine structures in the spectra of a field scattered by a cell can reveal information regarding nanometer-sized organelles that are well below the diffraction limit. Such information may in turn be used to monitor subcellular content and gain insight into normal and pathogenic phenotypes without perturbing the cell or its environment [1-5].
The first method to utilize the unique spectral features of light that has interacted with cells in order to recover structural information in vivo is light scattering spectroscopy (LSS) [1]. In this approach, measured spectra are analyzed to discriminate periodic fine structures that are well described by Mie theory, an analytical solution to the scattered field of a spherical scatterer using Maxwell’s equations. When applied to scattering by tissues, LSS must employ some method to discriminate contributions from singly scattered (nonrandomized) and diffusely scattered (randomized) fields; such methods include mathematical modeling [1], polarization gating [6], or coherence gating [7]. LSS has shown very promising results for diagnosing different types of cancers in vivo, and has hence received much attention as a unique clinical tool for noninvasive diagnosis. However, this method is not without its limitations. For example, LSS suffers from poor spatial resolution, since measurements are subject to spatial averaging over relatively large areas.
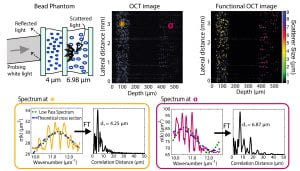
To address these limitations, we again leverage the capabilities of SOCT. In the example below, we obtain the spectrum from each point in the image using a modified short time Fourier transform, and then analyze different properties of the signals to assess the structure of the scattering objects. Specifically, we look at the wavelength-dependent scattering cross-section and other coherent effects, known as local oscillations. This information can then be used to render another image based on the size of the scatterers [8,9].
This method can be useful in medicine since the cell nuclei, which interact similarly with light as the bead phantom, are a useful biomarker of cancerogenesis [10].
References:
[1] Perelman, L., Backman, V., Wallace, M., Zonios, G., Manoharan, R., Nusrat, A., et al. (1998). Observation of periodic fine structure in reflectance from biological tissue: a new technique for measuring nuclear size distribution. Physical Review Letters, 80(3), 627–630.
[2] Backman, V., Wallace, M., Perelman, L., Arendt, J., Gurjar, R., Müller, M., et al. (2000). Detection of preinvasive cancer cells. Nature, 406(6791), 35–36.
[3] Itzkan, I., Qiu, L., Fang, H., Zaman, M. M., Vitkin, E., Ghiran, I. C., et al. (2007). Confocal light absorption and scattering spectroscopic microscopy monitors organelles in live cells with no exogenous labels. Pnas, 104(44), 17255–17260.
[4] Kim, Y., Turzhitsky, V., Liu, Y., Roy, H., Wali, R., Subramanian, H., et al. (2006). Low-coherence enhanced backscattering: review of principles and applications for colon cancer screening. Journal of Biomedical Optics, 11, 041125.
[5] Subramanian, H., Pradhan, P., Liu, Y., Capoglu, I., Rogers, J., Roy, H., et al. (2009). Partial-wave microscopic spectroscopy detects subwavelength refractive index fluctuations: an application to cancer diagnosis. Optics Letters, 34(4), 518–520.
[6] Backman, V., Gurjar, R., Badizadegan, K., Itzkan, I., Dasari, R. R., Perelman, L. T., & Feld, M. S. (1999). Polarized Light Scattering Spectroscopy for quantitative Measurement of Epithelial Cellular Structures In Situ. IEEE J Selected Topics in Quantum Electronics, 5(4), 1–8.
[7] C. H. Yang, A. Wax, I. Georgakoudi, E. B. Hanlon, K. Badizadegan, R. R. Dasari, and M. S. Feld, “Interferometric phase-dispersion microscopy,” Opt. Lett. 25(20), 1526–1528 (2000).
[8] Wax, A., Yang, C., & Izatt, J. (2003). Fourier-domain low-coherence interferometry for light-scattering spectroscopy. Optics Letters, 28(14), 1230–1232.
[9] Robles, F. E., & Wax, A. (2010). Measuring morphological features using light-scattering spectroscopy and Fourier-domain low-coherence interferometry. Optics Letters, 35(3), 360–362.
[10] Robles, F. E., Zhu, Y., Lee, J., Sharma, S., & Wax, A. (2010). Detection of early colorectal cancer development in the azoxymethane rat carcinogenesis model with Fourier domain low coherence interferometry. Biomedical Optics Express, 1(2), 736–745. http://doi.org/10.1364/BOE.1.000736